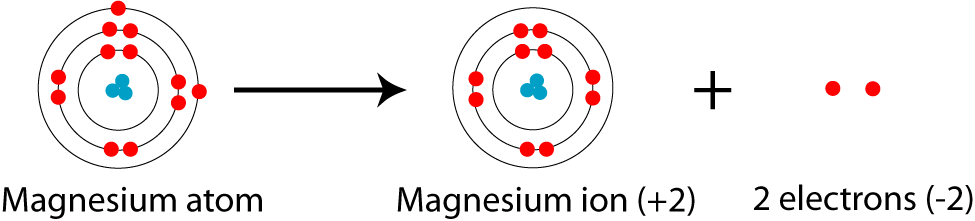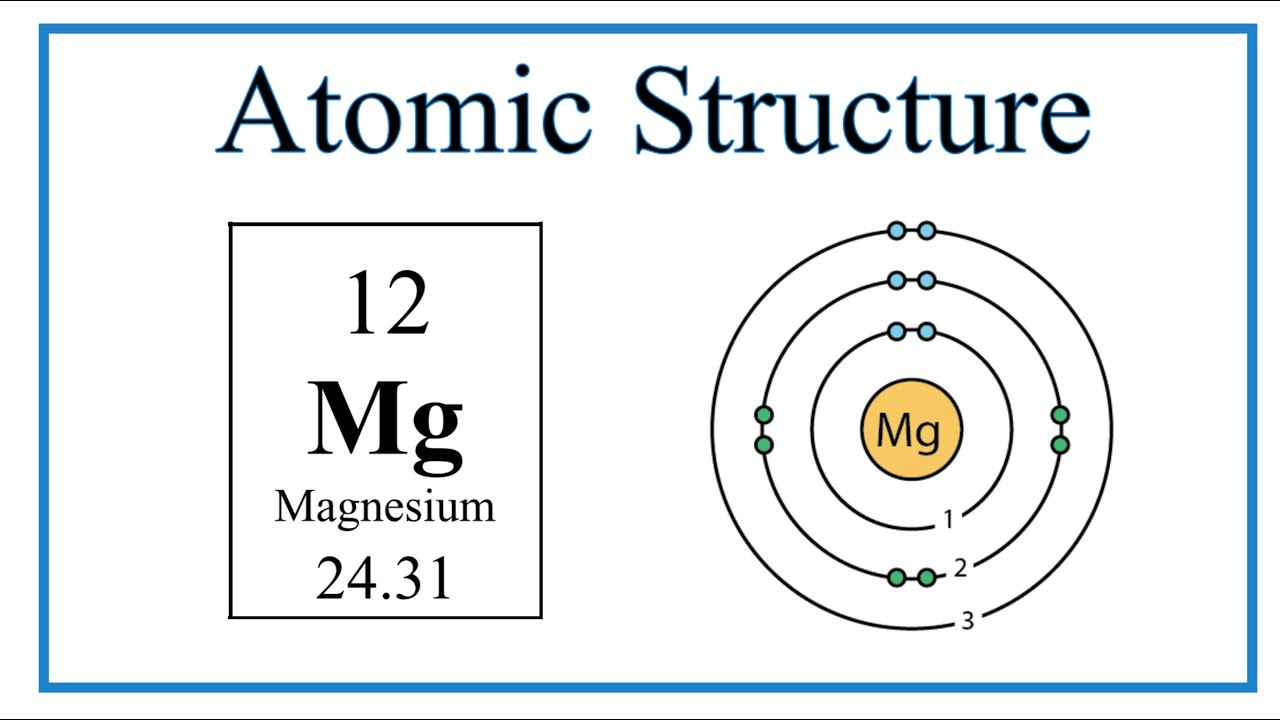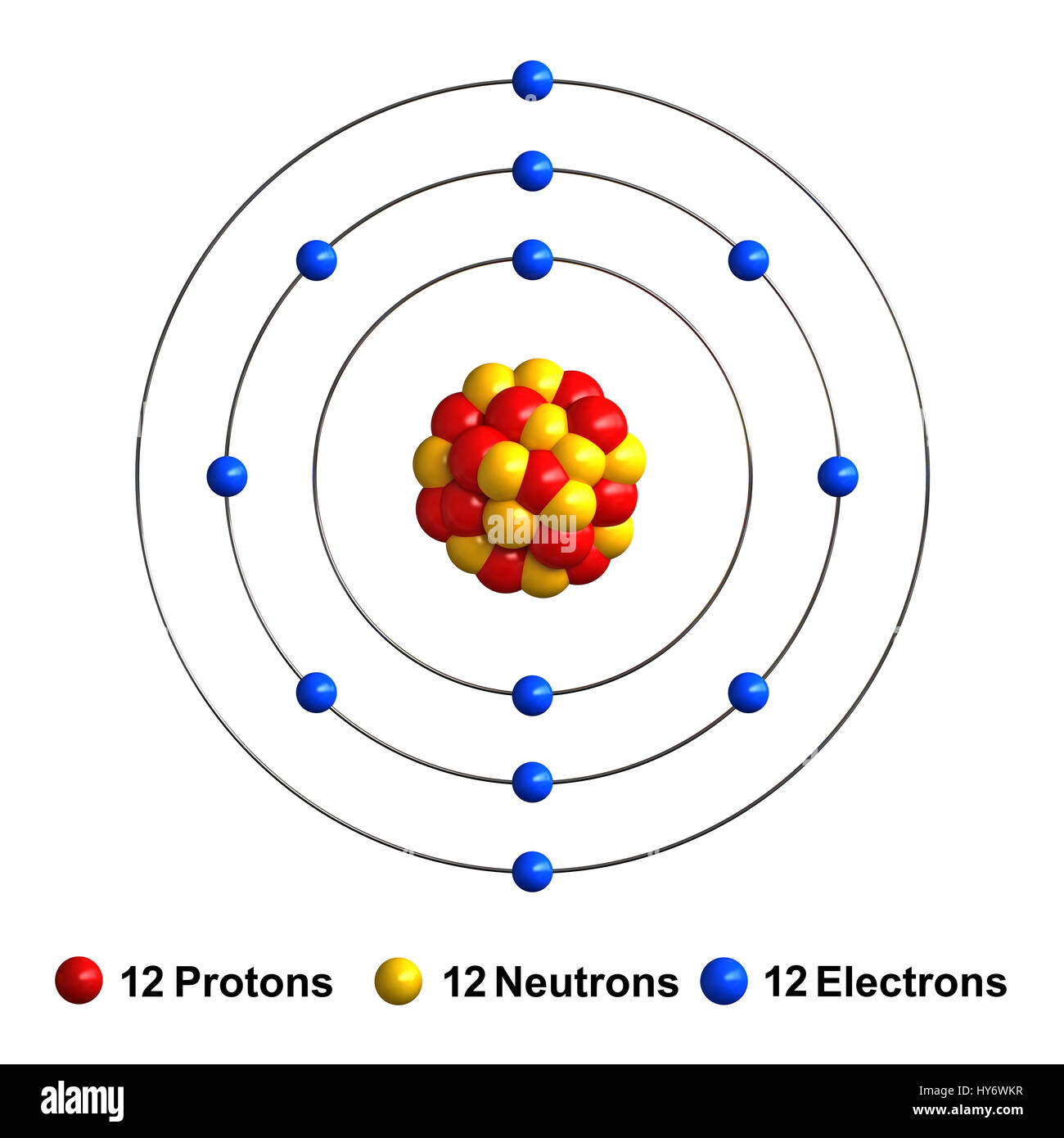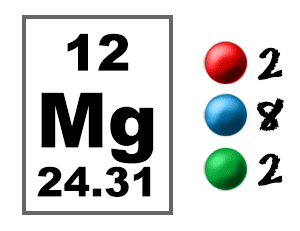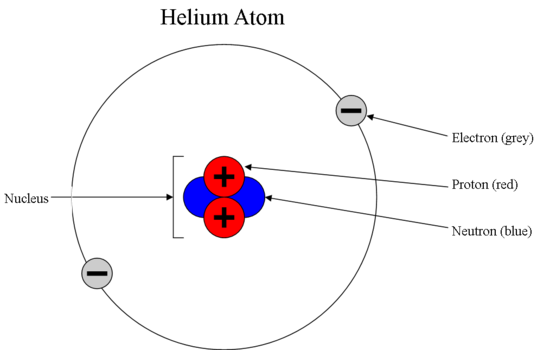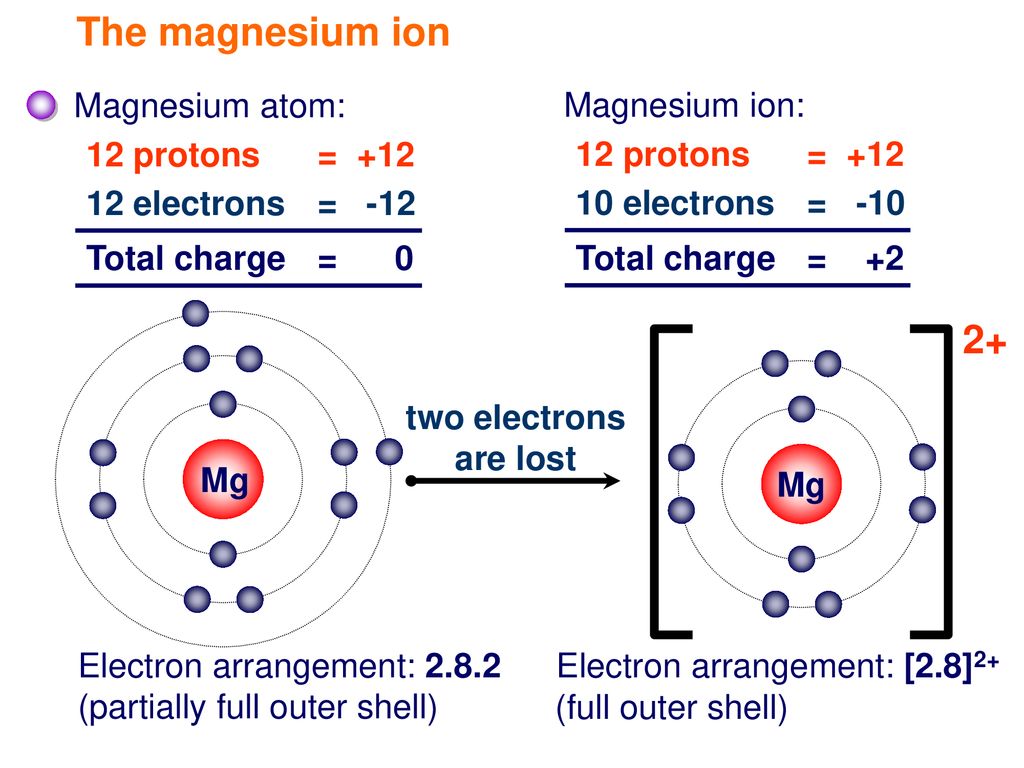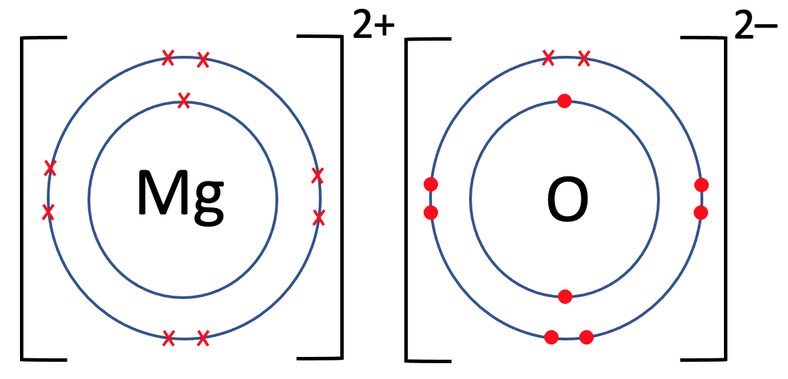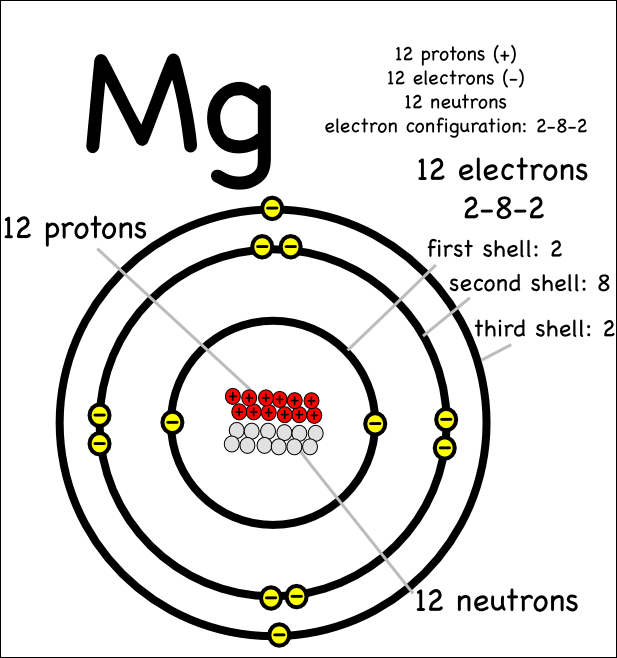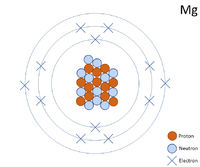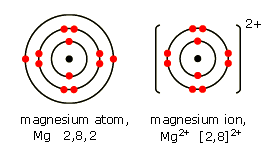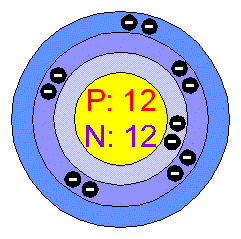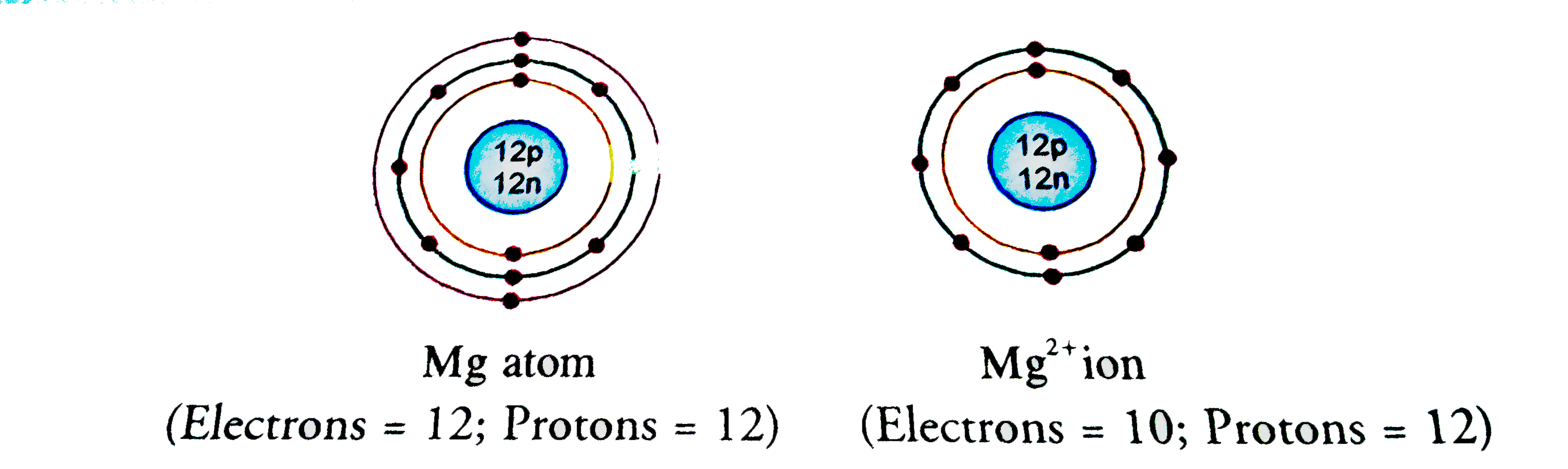
Show the electron distribution in magnesium atom and magnesium ion diagrammatically and also give their atomic numbers.
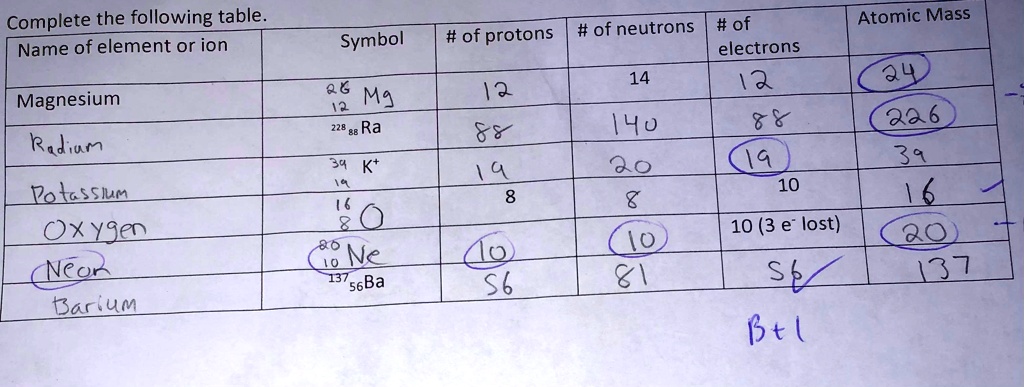
SOLVED: Complete the following table Name of element or ion #of neutrons #of #of protons electrons 14 12 12 140 8 Atomic Mass Symbol Magnesium Radiun 06 Mg ee Ra 226 3

Isotopes. Isotopes of Magnesium Atomic symbol Mg Mg Mg Number of protons Number of electrons Mass number Number of neutrons ppt download
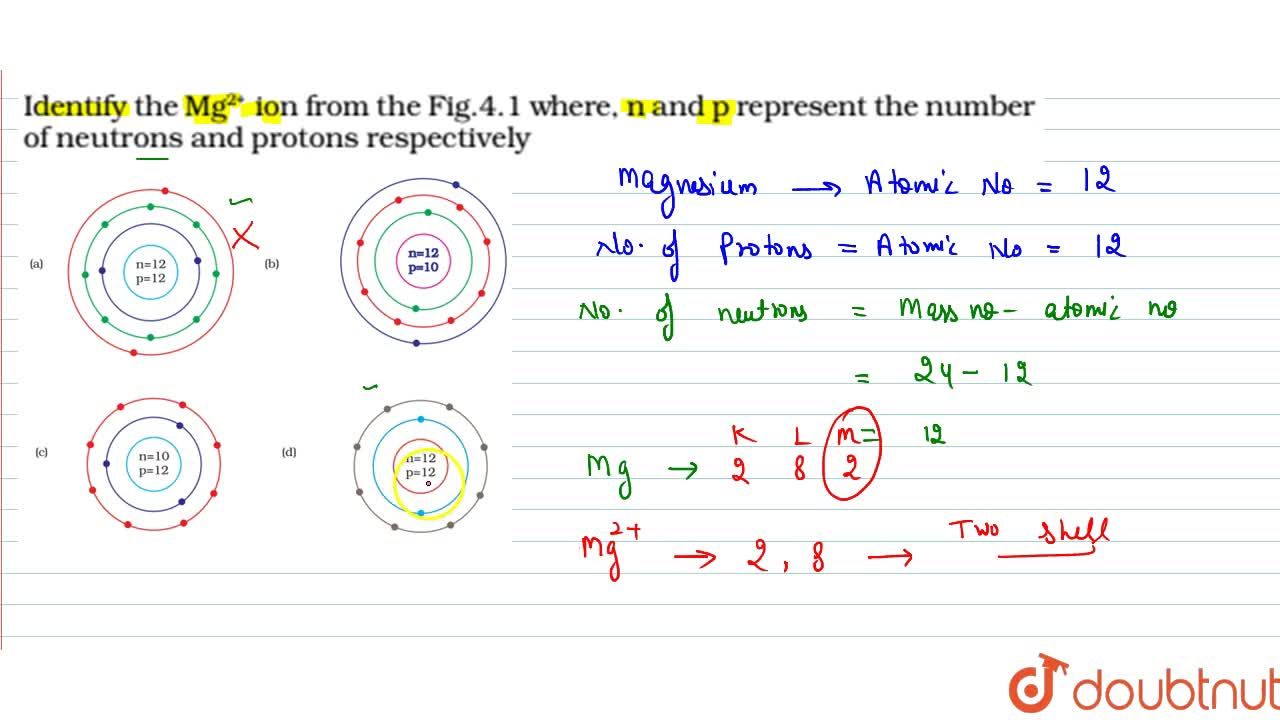
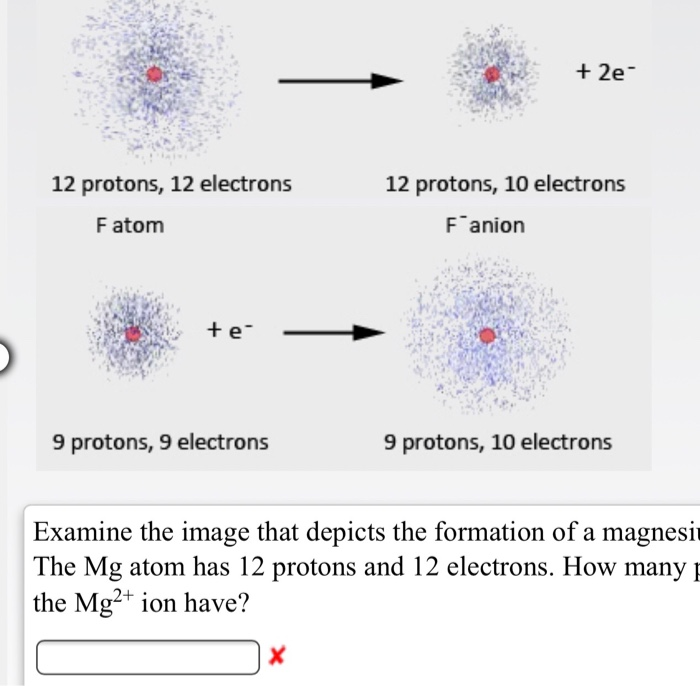
Identify the Mg^(2+) ion from the figure where, n and p represent the number of neutrons and protons respectively.

What is the Difference Between Magnesium Atom and Magnesium Ion | Compare the Difference Between Similar Terms

SOLVED: A magnesium ion; Mg2+, has: 0 12 protons 13 electrons 24 protons and 26 electrons 12 protons and 10 electrons 24 protons and 22 electrons 12 protons and 14 electrons

What is the Difference Between Magnesium Atom and Magnesium Ion | Compare the Difference Between Similar Terms